FidoCure® Targeted Therapies Early Data Shows Similar Adverse Effects to Palladia
To accommodate, we worked hard to move up the release date of our adverse effects data summary. Our data is based on 455 canine patients who enrolled in FidoCure-enabled targeted therapies between February 2018 and February 2020, with 390 of these whose clinicians reported outcomes .
Conclusion:
Our key conclusion is that the side effect profiles of these targeted therapies are very similar to Palladia (toceranib phosphate). Thank you to all of the veterinary oncologists who have opted for FidoCure® genomic sequencing, precision therapies, and graciously shared the outcomes of your cases. We have aggregated your data on several hundred dogs and are sharing this with you and your colleagues so that you are more empowered and informed when speaking to your clients. The data below is retrospective based on your medical records, clinician-reported outcomes, and completed Adverse Effect (AE) forms, along with client-reported outcomes.
As you can see from the chart below, the most common side effects involving diarrhea, vomiting, inappetence, and lethargy were mild. For the targeted therapies in our formulary, these side effects occurred in approximately 20% of patients for most of the drugs.
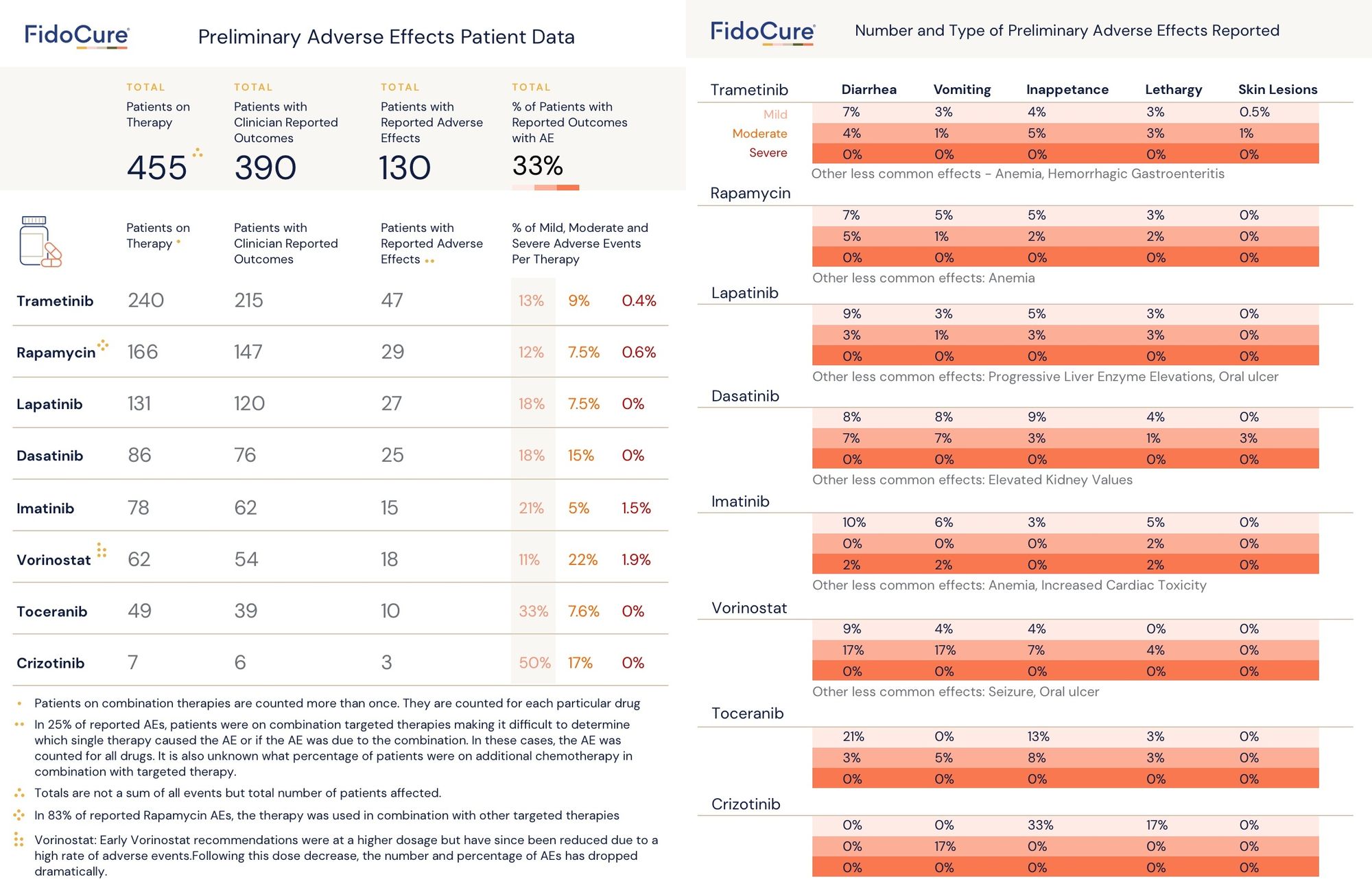
We are in the final stages of fully implementing the VCOG-CTCAE system for reporting adverse events. Until that system is in place, this three-tiered adverse event classification system was adapted by our team (Dr. Gerry Post, Lindsay Lambert, and Dr. Garrett Harvey) to retrospectively analyze the FidoCure® dataset.
Gradations are as follows:
- Mild: Asymptomatic or minimal symptoms; clinical signs or diagnostic observations only; minimal intervention, including drug holiday interventions 7 days or less and resolved when restarting therapy.
- Moderate: Minimal to medically significant symptoms that are not immediately life-threatening, outpatient or noninvasive intervention indicated; moderate limitation of activities of daily living (ADL), moderate decrease in quality of life (QoL). This may include discontinuation of therapy due to QoL.
- Severe: Medically significant including life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; significantly limiting ADL or QoL.
Further notes and limitations of the data: It is important to note that since this is real world evidence, many patients were treated with combination therapy, making it difficult to determine whether an adverse event was caused by one particular drug or the combination of therapies.
When evaluating the tolerability of new targeted therapies, toceranib was used as the baseline. The goal is to identify therapies that are at or below the number of reported AEs for toceranib. Moreover, toceranib is in the same class of drugs (tyrosine kinase inhibitors) as the majority of the drugs being evaluated. An important note is that the AE rate of toceranib may differ from the previously reported rate in prospective studies.
Moving forward, FidoCure® is shifting to prospectively collecting clinician-reported adverse events using our FidoCure® Adverse Event Case Report Form, in accordance with the standards set by the Veterinary Cooperative Oncology Group - Common Terminology Criteria for Adverse Events (VCOG-CTCAE).
FidoCure®’s goal is to partner with the veterinary oncology community to expand your toolbox of diagnostics, therapeutics, and information so that you can provide your clients with more options and your patients with the highest and most sophisticated level of care.
We value your input. Our goal is to improve the quality of veterinary oncology by bringing personalized medicine to the veterinary field and bringing much needed technology and capital to our profession. If you have any suggestions on how we can improve our services or products please feel free to reach out to me at gerry@fidocure.com.
This data is meant to be used for educational purposes only and no claims are being made.
Dr. Gerry Post
DVM · MEM · DACVIM