Personalized Medicine: Because Not All Tumors Are Created Equally
With new research and medical advancements, immunotherapy is the new frontier in veterinary oncology.
Please join us in welcoming Dr. Nicola Mason, B.Vet.Med, PhD, DACVIM (Internal Medicine), Professor of Medicine & Pathobiology at the University of Pennsylvania School of Veterinary Medicine. She is an innovator in veterinary immunology, leading clinical studies and research laboratories investigating canine oncology.
What do you mean by the title of this webinar, “not all cancers are created equally”? Why is it important for veterinarians and medical professionals to understand that concept?
We’re all in this together – to understand more about cancers and to develop better treatments. As a veterinarian, I think about this for dogs mostly, but we need to bridge the gap between the science and the clinic in both veterinary and human medicine.
In terms of patient advocacy, it drives home the importance of what we and others do in this field: not just for veterinary patients, but for children and adults, as well.
What do I mean by, “All tumors are not created equal.” In the clinic, you probably see dogs who come through with lymphoma, for example. You type them as B-cell lymphoma. Some of those dogs will respond well to CHOP induction chemotherapy and go into remission and stay there for 6-8 months, then relapse. Some of them don’t respond to chemotherapy, at all. And some of them respond to chemotherapy and stay in remission for 2-3 years.
We see this with other cancers, such as hemangiosarcoma (HSA). Most dogs with stage 2 HSA will have their spleens removed. Most of them will die within 6 months from the metastatic disease. There is a very small percentage of them that may go on for longer.
We know when we look at all these different tumor types, they don't just seem to be the same. There are different responses.
This is something that intrigues me: what is the difference between a dog that presents with stage 2 HSA who dies quickly, versus those who survive for a long time.
If you examine the slides, it speaks to what I’ve been referring to. Is it a tumor difference but at a deeper level?
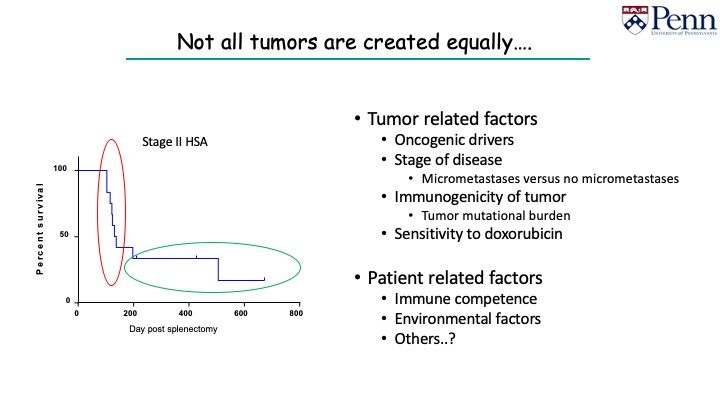
With the advance of next generation sequencing (NGS), we can investigate this concept with tumor-related factors:
- Perhaps different drivers give tumors with different prognosis
- Certain oncogenes may be more aggressive than others
- Long-term survivors do not have micrometastasis
Stages of the diseases can be different, but there are also patient-related factors: immune competence, environmental factors, and perhaps others.
All of this is what I think about when I say they are not all created equally. And this is what we’re trying to understand now.
You published two wonderful papers on the genomics of hemangiosarcoma in dogs as models for angiosarcoma in people. Can you discuss those findings? Why do you think they are important for medical doctors? Veterinarians?
We’ve had an interest in comparative oncology at Penn. You read the literature and there’s a tumor type in people called angiosarcoma. People have tried to make the connection that HSA and angiosarcoma are quite similar. They look similar under a microscope, but that’s quite superficial.
We’ve started a collaboration with David Roth and Guannan Wang to try to understand at a molecular level whether they share similar molecular features.
Angiosarcomas is not a good tumor to have in people. Fortunately, it’s extremely rare. In a majority of people, it is cutaneous. A small percentage of them are visceral.
We use HSA as a parallel patient population with visceral and cutaneous angiosarcoma in humans. We looked at HSA and asked the question, “Are these tumors mutated? Are there druggable targets that can be relevant?”
If there are a lot of mutations in the DNA of these tumors, they could be immunogenic.
For example, in the graph, PIK3CA was mutated in half of those dogs. We found the other half of the dogs did not have those mutations. They had mutations in other genes, similar to angiosarcoma.
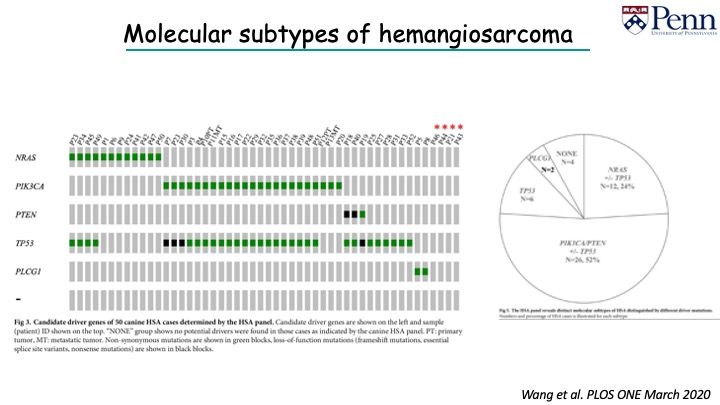
We did NGS and we were able to pick up additional mutations. We found a quarter of dogs of an expanded cohort had mutations in NRAS. Those dogs with NRAS mutations did not have mutations in PIK3CA; the two mutations seem to be mutually exclusive.
You can start to see that there seems to be different molecular subtypes of HSA. With NGS, we see there are differences in tumor associated genes.
Our two papers hone into these concepts. Mutations in these genes have been identified in HSA. You can now look at druggable targets and inhibitors for canine hemangiosarcoma, and perhaps, you can, if they work, turn them over to the human world for angiosarcoma.
The value of precision medicine is understanding your enemy: knowing what is going on and who can be treated with different inhibitors. Currently, what we do is that we treat everyone the same.
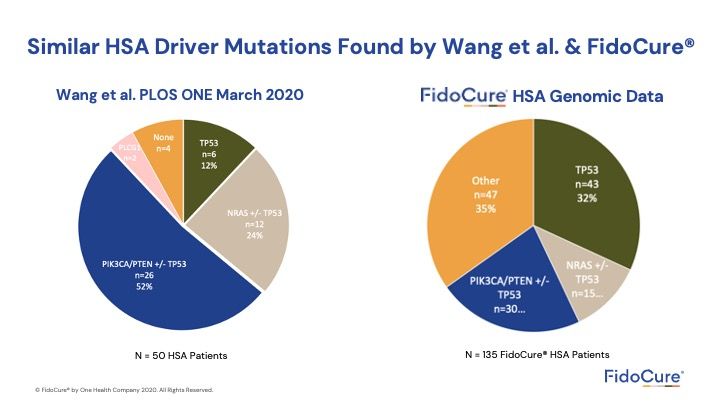
We examined 50 dogs. FidoCure® examined 150 dogs and is seeing the same thing. The benefit is that, now, targeted inhibitors are available, and we should be considering this.
Your research interests seem to span both immunology and genomics, is this because they are related or because you have an interest in both disciplines?
My love is this concept of being able to use the immune system to target cancers. To me, it’s so elegant. This system has evolved over tens of thousands of years. It’s evolved to recognize foreign things, like pathogens, bacteria, and abnormal cells. Taking this system that is finely tuned and honed to tweak it more to make it more effective to eliminate tumors that circumvent it. It’s a great concept.
Tumors in general arise from our own cells. It’s part of the problem.
We’ve been looking to see how foreign tumors really appear to the immune system and how many mutations it has in the DNA. Your immune system can recognize single amino acid changes as abnormal or foreign, and should be eliminated.
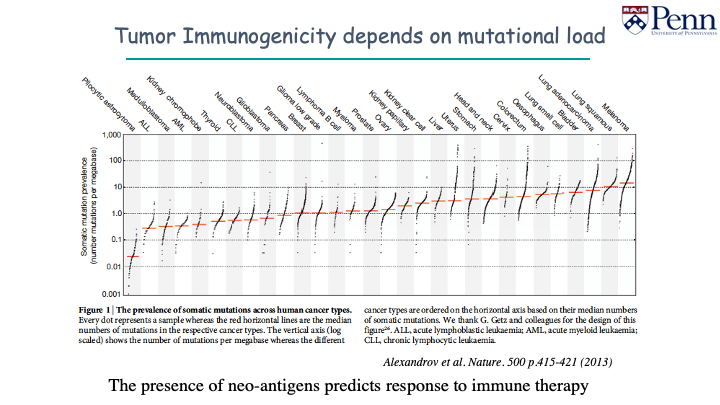
Melanoma, pulmonary carcinoma have lots of somatic mutations. Childhood cancers have low somatic mutations, and will look a lot less immunogenic. There are less mutations for the immune system to recognize, and more difficult to target with immunotherapy.
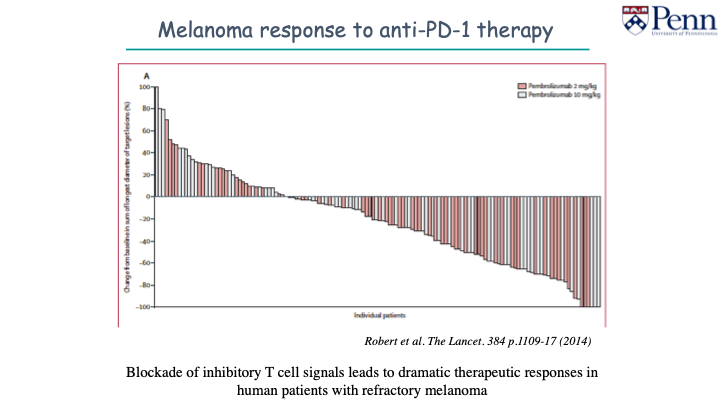
Immuno-oncology, or the harnessing of the immune system to treat cancer, is both a very old and a very new idea. What are you most excited about?
There is so much happening and it is incredibly exciting. I think we are already seeing dramatic responses in humans.
No one likes to say cure. At some point, people will start to use that word.
The checkpoint inhibitors are very interesting. The jury is still out on how dogs will respond to monotherapy. I think there is a role for this in combination therapies.
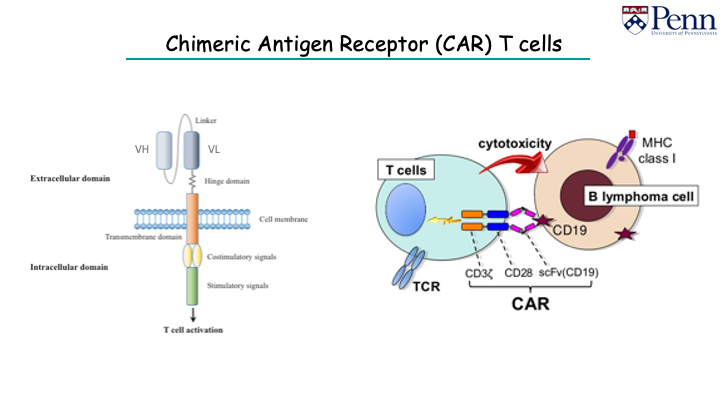
I’m also excited about chimeric antigen receptor (CAR) T cells - reprogramming those killer cells to recognize antigens of interest. We are cautiously optimistic we can get these to work.
A quick aside: there is a documentary called Breakthrough about Jim Allison, the individual who discovers the role of CTLA-4 in turning off T-cell responses. It's worth a watch.
When Dr. Cheryl London spoke at our webinar, she talked about the value of combination therapy and multi-modal therapy. What are your thoughts?
I agree with her. It’s unlikely we will cure cancer with a single approach. Combinations will help expand this to some patients.
Combination therapy of chemotherapy and targeted therapy makes sense.
Combination therapy of chemotherapy and immunotherapy on the surface doesn’t make sense because we think of chemotherapy as a suppressor. However, this doesn’t seem to be the case at all. We don't understand enough about immunotherapy and the effects of chemotherapy on the immune system. I think we are seeing now that the combination is actually more effective than immunotherapy on its own in some instances.
Of course, we know tumors mutate and there is inherent genetic stability. Being able to predict or recognize which types of tumors these patients have and using a combination of therapies in, hopefully, a nontoxic way will make sense .
Q&A
Are you able to get back to your lab now, even with COVID-19? Are you doing COVID-related research?
I am back in the lab. The lab closed down for 3.5 months. It was very difficult because experiments and clinical trials had to stop. We went to 20% capacity at the end of June, and now we’re at 50% capacity. We’re waiting on the call to move forward to 100%, but our clinical trials are up and running again.
It’s similar across the board to a lot of the academic sites. It’s been a tough time.
We aren’t doing COVID-related research.
The innovations you're talking about, in terms of your work in particular, when do you think they will be available to practitioners in the clinic?
You can argue they're already available. If you have a patient you think is eligible and they can come on to the clinical trials, you can have access now. We have a veterinary investigation center. I’m happy to talk to you about it.
In terms of making it commercially available, we’re still a ways off. I don't want to put anything out there until I have a degree of confidence it will make a difference and it will work. The other thing to mention is that with these powerful immunotherapies, you will see more side effects. It’s a toss up.
You have to think about it in terms of risk benefit. The humans patients who do well with CAR T-cell therapy experience cytokine storms. If you get through that, you can have a durable remission.
Everyone must be aware of the side-effects and how to manage them. The goal is to put something out there to induce and maintain remission.
Dr. Mason's presentation and data may be downloaded here.
To download the audio only version click here.
We’d like to take a moment to recognize everyone on the frontlines seeing patients and academics continuing research in the lab. You are truly the unsung heroes.
We’d like to thank you from the bottom of our hearts for continuing your work in these tumultuous times.
If you would like to get our webinar invites, please send us a note using our veterinarian contact us form.


